Avaximab™ Recombinant mAbs
- Avaxia has identified the structural basis for the ability of milk-derived antibodies to survive in the GI tract
- Key characteristics of Avaximabs
- Key structural attributes responsible for protease stability have been defined
- Analysis and plans for product development and pipeline in place for review
- Additional details available under NDA
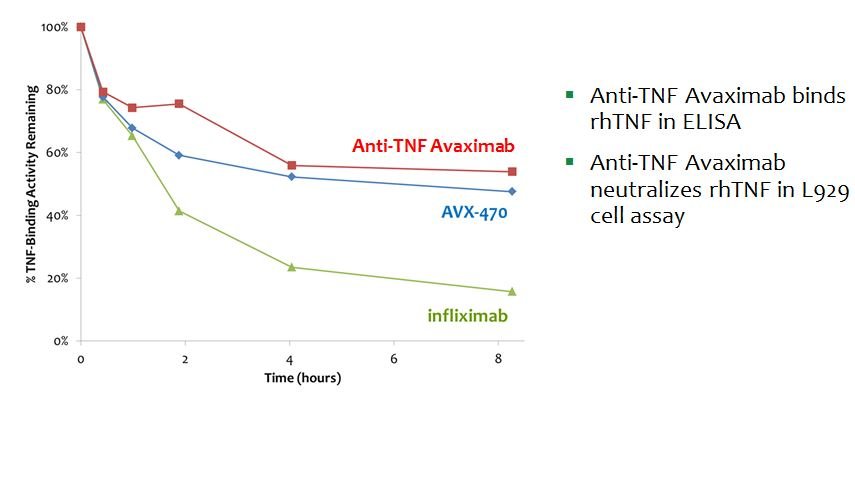
- Anti-TNF Avaximab Shows Stability Similar to AVX-470 in Digestion by Intestinal Proteases
- Avaximab-TNF: Neutralizes TNF and is Stable to Digestion
-
Stable to intestinal digestion
-
Minimal systemic exposure
-
Penetrate into inflamed gut mucosa
-
Potential for local activation of effector functions
-
Oral administration with twice daily dosing and superior with no “black box” warning
-
Efficacy comparable to injected anti-TNFs for inducing remission in moderate to severe IBD
-
Superior efficacy to injected anti-TNFs for maintaining remission in moderate to severe IBD
-Longer response time and fewer relapses than with parenteral anti-TNFs (20% per year currently lose anti-TNF response)
-No side effects that compel cessation of treatment
-
Chimeric antibodies produced that display increased resistance to proteolysis
Our lead program is Avaximab™-TNF for the treatment of inflammatory bowel disease (IBD).
IBD encompasses two related but different diseases: ulcerative colitis and Crohn's disease. These serious diseases cause chronic inflammation of the intestinal tract, resulting in a significant reduction in quality of life and the risk of life-threatening complications and diseases, including cancer. More than 2.5 million people worldwide have these diseases.
Avaximab™-TNF is an orally administered recombinant anti-TNF antibody that acts locally in the intestines where IBD occurs. Systemically-delivered, anti-TNF antibodies have proven effective for the treatment of IBD, but they can cause infrequent but serious side effects such as opportunistic infections and certain cancers when they dampen the immune system. Effective long-term treatment can also be limited by issues related to immunogenicity. As a result there is an important unmet medical need for IBD treatments that are as effective as currently marketed anti-TNFs but which have a reduced risk of side effects and immunogenicity. Avaxia believes that Avaximab™-TNF has the potential to fulfill this need, potentially expanding the anti-TNF market well beyond its current $2.5 billion in annual sales.
Clinical Trial Results
In 2014, Avaxia completed a first-in-human clinical trial of AVX-470 in ulcerative colitis patients. This trial was a double-blind, placebo-controlled, ascending dose study of AVX-470 in adult ulcerative colitis patients. Three different dose levels of AVX-470 were administered for a period of 28 days. The study involved 13 trial centers in the U.S., Canada, Belgium and Hungary. A total of 36 patients were treated in the trial, with nine patients receiving placebo and 27 patients receiving the drug. The trial successfully met its primary and secondary objectives (see Press Release).
- Safety: No product-related safety or tolerability concerns were identified
- Distribution: Bovine antibodies were present in colon tissue and stool; levels in serum were no higher than background
- Anti-Drug Antibodies: The measured antibody to AVX-470 was no higher than background in serum
The trial also included a number of exploratory assessments, which included the evaluation of clinical and endoscopic responses and effects on biomarkers. Although limited in patient numbers, AVX-470 showed dose-dependent favorable trends across multiple clinically important endpoints, including patient response and remission, as well as reduced serum C reactive protein (CRP), serum IL-6, and tissue TNF levels. These signs of biological activity and proof of mechanism are highly encouraging, especially as they were observed after dosing a small number of patients after only four weeks of dosing.
Avaxia disclosed detailed trial results at the 2014 United European Gastroenterology Week and the 2014 Annual Scientific Meeting of the American College of Gastroenterology (see Technology, Publications).